
Are there ever nuclear reactions happening in our bodies?

- Related Topics:
- Quirky questions,
- Molecular biology,
- Physics
“Nerdy high schooler” from California asks:
“Are there ever nuclear reactions happening in our bodies?”
Yes! There are plenty of nuclear reactions happening in our bodies all the time!
Unfortunately our radioactivity doesn’t give us superhero x-ray vision or anything. But it doesn’t harm us either. Mostly, the nuclear reactions are too few and too weak to even interact with our biology.
But it might be a surprise that there are nuclear reactions happening all the time. To understand these nuclear reactions all depends on what we are talking about when we say nuclear reaction.
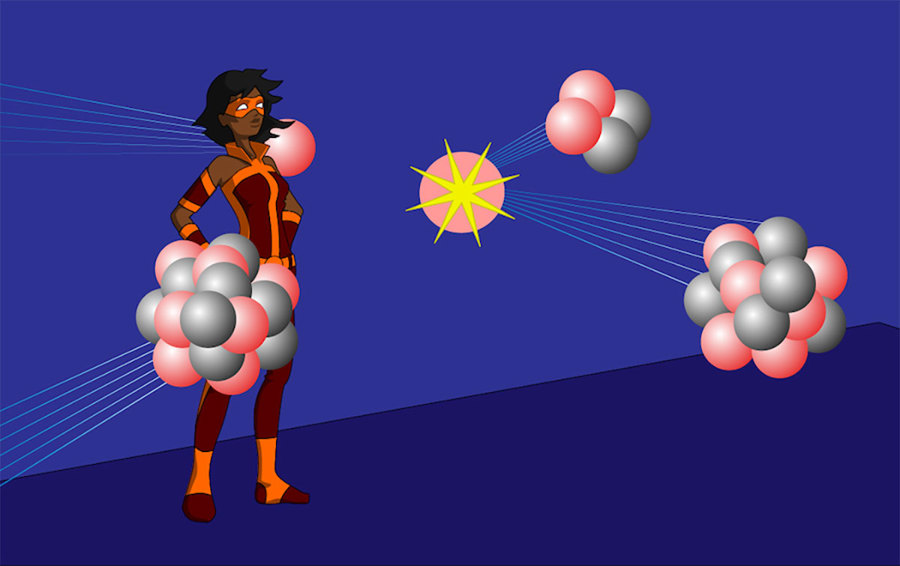
There are many types of nuclear reactions
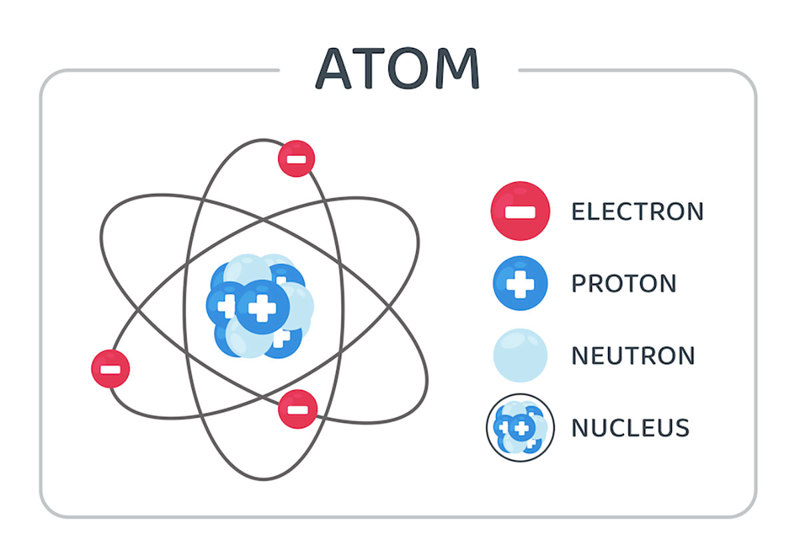
Everything we are familiar with in our lives are composed of atoms. But what are atoms made of? Atoms are made of electrons, protons, and neutrons. The number of protons in an atom defines what type of element the atom is.
Familiar elements (like oxygen, hydrogen, carbon, or nitrogen) can be mixed together to form more complex things. Two hydrogen and one oxygen makes water. 12 hydrogen, 6 carbon, and 6 oxygen? Now you have sugar. Mix the water and sugar together and you are well on your way to a cake.
The protons and neutrons of an atom are located together in the middle (or nucleus) of the atom. Any time the number of these sub-atomic particles changes, it causes a nuclear reaction.
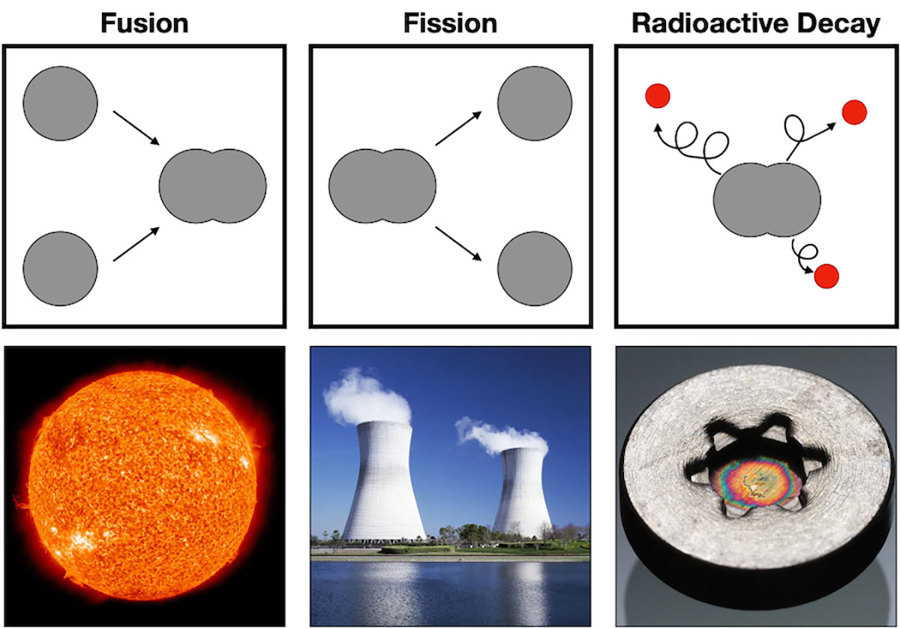
Fusion
The easiest place to look for a nuclear reaction is straight up. Yep, the sun is constantly changing elements into different elements, and making a lot of energy in the process. The intense heat and pressure inside stars like the sun make it possible to fuse nuclei together to make bigger, more massive atoms.
This reaction releases lots of energy, which we see and feel as light and heat. However, these nuclear fusion reactions can’t happen without the intense heat and pressure of a star. So it’s pretty safe to say that there are no fusion reactions happening in our body.
Fission
Fission reactions happen when the number of sub-atomic particles of an atom is reduced. Nuclear power plants take the element uranium and split it into lighter elements (such as cesium and rubidium), releasing energy in the process.
Like fusion reactions in the sun, it takes very specific conditions to sustain a nuclear reaction like this. For starters, uranium is the only natural element on earth that can be used as reactor fuel. Furthermore, it takes specialized human-made elements like plutonium to get these reactions started. Since our bodies don’t have extra stomachs filled with plutonium, there are never fission reactions happening in our bodies.
Radioactive Decay
What nuclear reactions do happen in our bodies then? Radioactive decay! This is when elements spontaneously lose small particles.
While every element is defined by the number of protons in the nucleus, the number of neutrons can vary. These different variations are called isotopes. And since some isotopes are more stable than others, a fairly common reaction is where an unstable isotope loses neutrons to reach a more stable state.
These unstable isotopes are what we typically describe as “radioactive”. When radioactive isotopes lose neutrons, they release a bit of energy in the process.
Every element has at least one radioactive isotope. For the most part, those isotopes are pretty rare compared to the more stable versions. That said, there are a small number of radioactive isotopes among the elements that make up our bodies!
The radioactive isotopes in our bodies
The elements carbon, hydrogen, oxygen, or nitrogen make up about 96% of our body. The radioactive isotopes of these elements are very rare in nature. But on the other hand, we have a lot of these elements in our bodies. So overall there are a few thousand radioactive decays per second from elements like carbon1,2.
However, the type of radiation that is emitted when these isotopes decay is very weak. Typically, it doesn’t do any damage to the body.
The most significant source of radioactive decay in our bodies is from potassium, which makes up about 0.2% of the body. Potassium is important in everything from neurons functioning to a stable pH in the blood. While it is a less common element than carbon, unstable potassium isotopes decay differently, emitting gamma rays. Gamma rays can be more harmful than other types of isotope radiation.
One estimate of human radioactivity is that about 5000 potassium atoms undergo radioactive decay each second2,3,4. While this may sound like a lot, the total amount of energy this emits is still pretty low. It’s still well below an amount that could make you sick.
Other sources of gamma rays in our bodies include isotopes of uranium, lead, thorium, polonium, or radon. These can enter our bodies through our diets or as we breathe. But they’re typically found in us at low levels that really don’t affect our biology.
This kind of baseline information about standard nuclear reactions in our body is pretty important to know. After all, it would be pretty confusing to try to eliminate radiation hazards without knowing the baseline value your detectors show from just humans in the room!
Not only that, we need to know what amounts of radiation are ok so that we can keep people safe from nuclear radiation hazards. This is actually an entire field of study called medical physics.
Are the nuclear reactions happening in our body ever unsafe? Sometimes, living near large sources of radioactive isotopes (like uranium mines or places with a specific type of bedrock) can lead to isotopes building up in your body to too high amounts. For example, many public health agencies encourage testing for radon. This can be done with relatively inexpensive and widely available test kits.
Overall, the nuclear reactions happening inside our body can pretty much be ignored. But that isn’t to say there aren’t any happening at all. So, if you are feeling a little sentimental, wave goodbye to a gamma ray; odds are there is one leaving one of your potassium atoms right now.
Read More:
- Youtube: What Is Radioactive Decay?
- Health Physics Society: Radioactivity and Health FAQs
- American Association of Physicists in Medicine: What is Medical Physics?
- EPA: Radon safety

Author: Dr. Ian Heller
When this article was published in 2022, Ian was a recent Ph.D graduate from the Stanford Department of Developmental Biology. His thesis work focused on the genetics of injury repair.
 Skip Navigation
Skip Navigation
