How does the DNA helix lose bases?

- Related Topics:
- Mutation,
- Molecular biology
A curious adult from Ghana asks:
"Why is depurination more common than depyrimidination?"
Our cells actually lose bases in our DNA all the time! Although our cells can fix most of these mistakes in our DNA easily, it’s important to understand why these mistakes happen since they can lead to DNA damage.
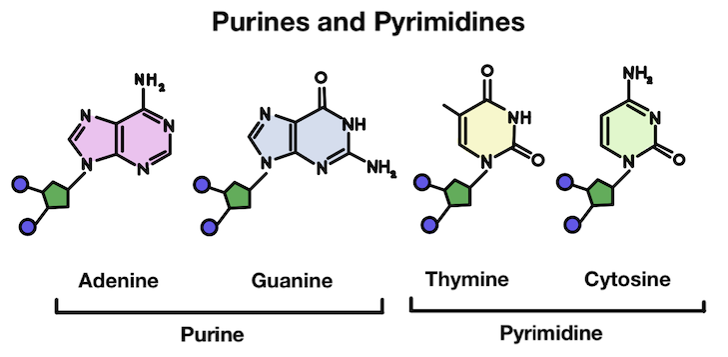
Our DNA is made up of four different bases: adenine (A), thymine (T), guanine (G), and cytosine (C). These four bases have slightly different structures. Adenine and guanine are purines, which are slightly larger. Thymine and cytosine are pyrimidines, which are slightly smaller.
It's pretty common for our DNA to become damaged. One type of damage is for the base pair to fall off the helix backbone. Losing an A or a G is called depurination. Losing a T or a C is called depyrimidination.
In order to understand why losing purines is more common than losing pyrimidines, we’ll need to get into some specifics on the chemistry of how our DNA is assembled and disassembled!

Purines vs. Pyrimidines
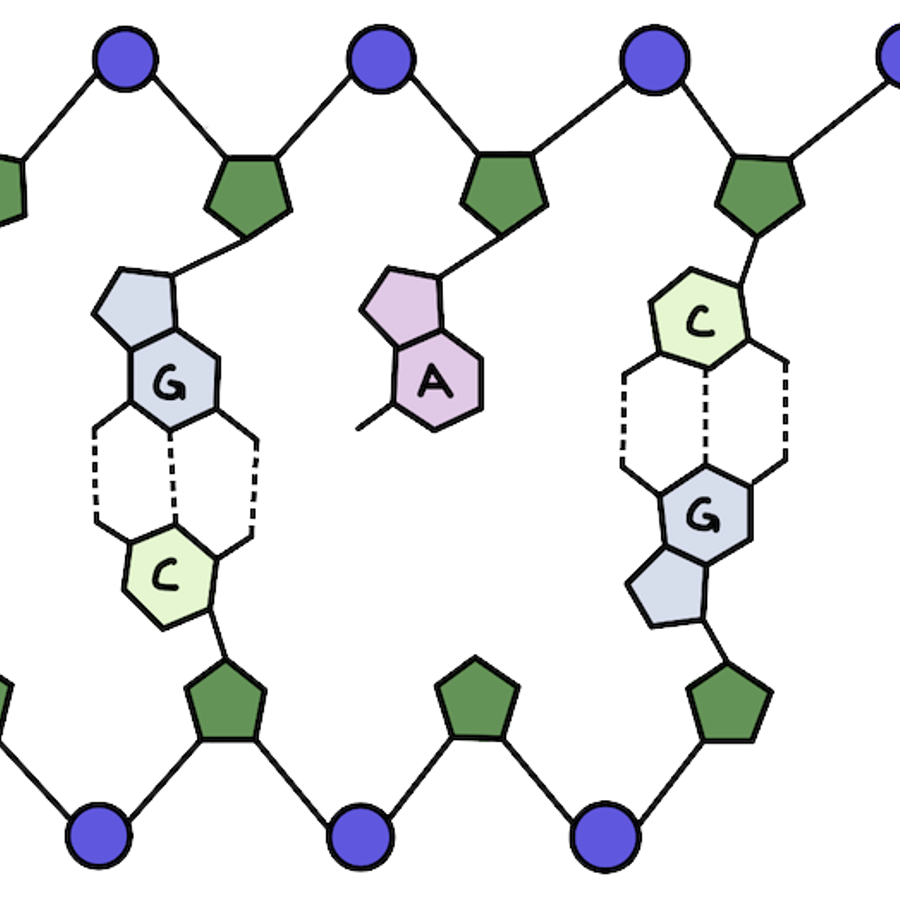
What makes up our genetic material? Our DNA has a double helix structure. There are two strands of DNA that are linked together and wind around each other, kind of like a twisted ladder.
Let’s zoom in: what makes up each strand of our DNA? The outside is made up of alternating phosphate and sugar groups that form the backbone. On the inside of the helix you'll find the base pairs that make up the information in our DNA (those A’s, T’s, C’s, and G’s!).
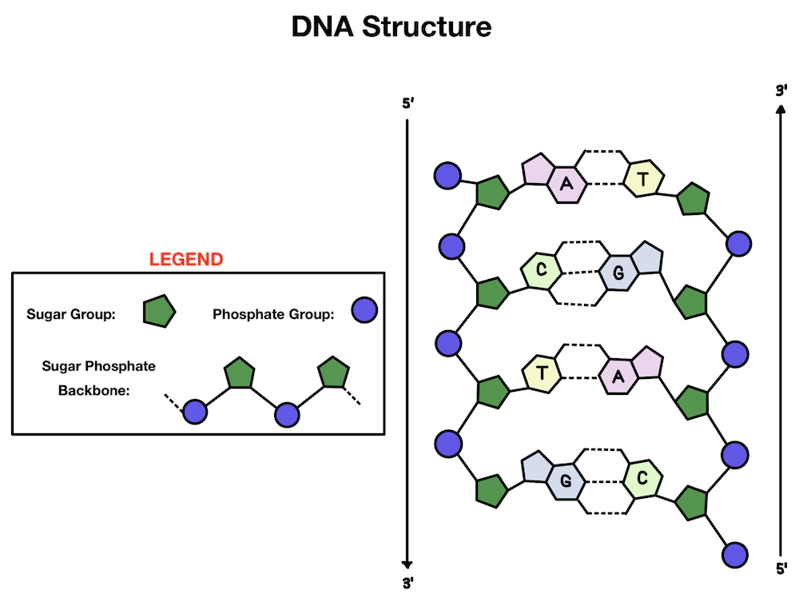
The base pairs make up the genetic information found in our cells: DNA and RNA. While the other parts of DNA are there for structural support, base pairs actually make up the genetic code that makes each of us who we are!
Adenine (A) and guanine (G) are purines, while thymine (T) and cytosine (C) are pyrimidines.
Base Structure
Do you see anything interesting about purines and pyrimidines? They’re grouped into these two groups because thymine and cytosine are much more similar in structure to each other than the other bases. The same goes for adenine and guanine!
Purines are made up of two rings, while pyrimidines only have one. In chemistry, rings are what we call a closed circular arrangement of atoms. These two different structures partially explain the reason why depurination is more common than depyrimidination!
The purines are much more complex and large, making them more susceptible to removal from the DNA strand.
Spreading out charge instability is the key!
While our DNA is very stable, certain types of chemical reactions can cause bases to fall off the sugar backbone. This can happen when the link between the base and sugar is broken.
The bond between the sugar and the base can be spontaneously broken by water molecules. Water is H2O, which easily floats around as H+ and OH-. The positively charged H sometimes become attracted to negatively charged things in a cell.
Whenever a positively charged H attaches to a chemical structure, that chemical structure is more unstable. Chemical structures are typically neutral. An extra positive or negative charge is like an unwanted pest! The chemical structure will usually try to get rid of the unwanted charge, even if that means breaking apart.

The image above shows what happens when a hydrogen gets wedged in between a base and the backbone. But these unwanted hydrogen atoms don't just get added at random. It's pretty rare for a hydrogen to get added directly to that spot.
Instead, it usually attaches to the strongest negative charge in the structure. That's because hydrogen has a positive charge and opposites attract! Which spot is most negative (and has the most free electrons) has to do with how the different atoms interact.

The base is disrupted after the hydrogen attaches. There is a positive charge!
In the pyrimidine, the hydrogen attaches to an oxygen. But since the oxygen is hanging off the ring, the positive charge stays outside the main structure. Usually, it just falls back off without causing any problems.

But in purines, the hydrogen attaches to an atom within a ring structure. Because it's added inside the ring, the unwanted positive charge becomes shared by several atoms in the purine base structure. The extra charge is also shared by the connection point to the DNA backbone, making it unstable.
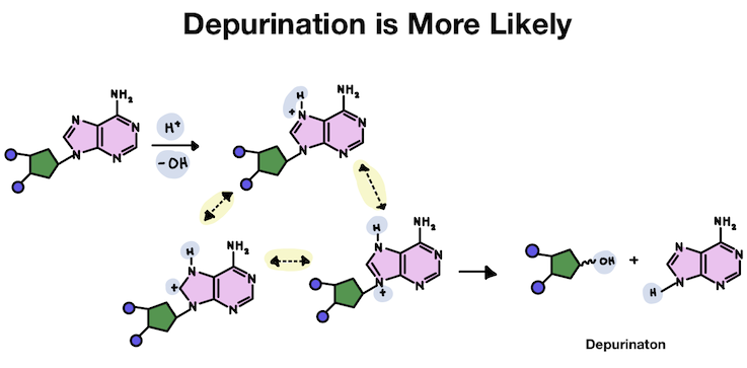
Since the hydrogen and the positive charge is able to stay on the purine for much longer than the pyrimidine, the chances that the purine falls off from instability is much greater!

Author: Daiyao Zhang
When this answer was published in 2023, Daiyao was a PhD candidate in the Department of Chemical Engineering, studying the effects of matrix stiffness on pancreatic cancer chemoresistance in Sarah Heilshorn’s laboratory. Daiyao wrote this answer while participating in the Stanford at The Tech program.
 Skip Navigation
Skip Navigation
